CLINICAL RESEARCH
The GIE Nancyclotep has established a clinical research unit dedicated to the development of clinical trials in the field of nuclear medicine and, more recently, in therapy.
This initiative aims to support clinical research projects and play an important role within the Nancy University Hospital (CHRU).
This unit is equipped with modern technologies, ensuring optimal conditions for conducting both industrial and academic studies. By combining scientific expertise and cutting-edge equipment, this unit has become a key player in the development of clinical trials in nuclear imaging in France.
Nuclear Medicine Department
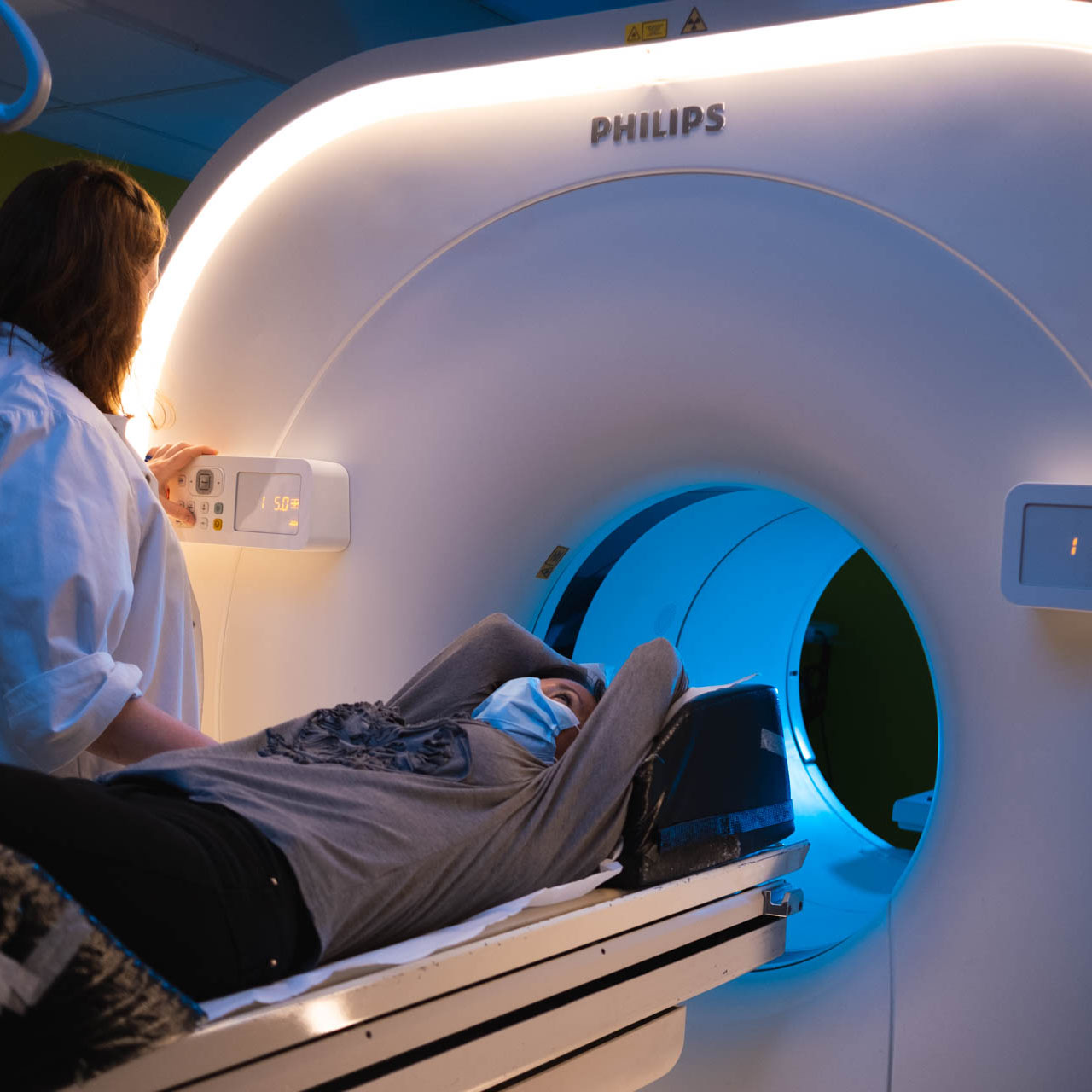
The clinical studies take place in the nuclear medicine department located at CHRU of NANCY-Brabois.
This department's infrastructure allows for conducting diagnostic and therapeutic clinical trials (Theranostics, more commonly referred to as Radioligand Therapy RLT) from phase 1 to phase 4.
The nuclear imaging department boasts advanced technical facilities with the following equipment:
The nuclear medicine department of CHRU Nancy, in collaboration with Nancyclotep, has become a major player in the development of RLT in France over the past few years, treating patients from the entire Grand Est region as well as from the Paris area.
From Diagnosis to Therapy
The nuclear medicine department at CHRU Nancy has 14 therapy rooms, 7 of which are lead-shielded, all dedicated to therapy for patients receiving Radioligand Therapy (RLT) treatments, located on the 11th floor, adjacent to the endocrinology department.
These rooms have been designed to offer a secure environment for administering radiotherapeutic treatments. The lead shielding of these rooms ensures optimal protection against radiation, for both patients and medical staff.
Treatments are administered to patients daily in this department. This facility ensures the rigorous administration of treatments while guaranteeing the safety of both patients and healthcare staff. This dedicated area makes CHRU Nancy a reference center for radioelement-based therapies that require strict radiation protection conditions.
Who is our team?
1
medical team
(11 doctors
et 34 radiology
technicians)
1
coordinating
project manager
1
regulatory
project manager
4
clinical research
technicians
2
radiopharmacists
1
medical physicist
A Technical Environment Dedicated to Clinical Trials
Within Nancyclotep, the clinical research unit has a RIA (Radioimmunoassay) laboratory. This laboratory meets the needs of academic and industrial sponsors for early-phase clinical trials, in compliance with regulatory standards.
This equipment includes:
The environment allows for the performance of:
Clinical research technicians (CRTs), specifically trained in the handling of radioactive biological samples, are authorized to collect samples directly from patients.
They then process the biological samples according to clinical study protocols, before on-site storage and/or shipment to partner central laboratories.
Scientific Output
Since 2020, the number of publications has reached 200.
Who are your contacts?
Our expert team is your primary contact for setting up your industrial clinical trials within the department. We assist you with:
Our team also supports doctors with their CHRU Nancy-sponsored trials, from a scientific idea to the publication of a scientific article.
Are you a trial sponsor?
Are you looking for a French nuclear medicine center for your clinical trial? Send your feasibility request to our team:
Véronique ROCH : v.roch@chru-nancy.frAnne-Sophie HUE : a.hue@chru-nancy.fr